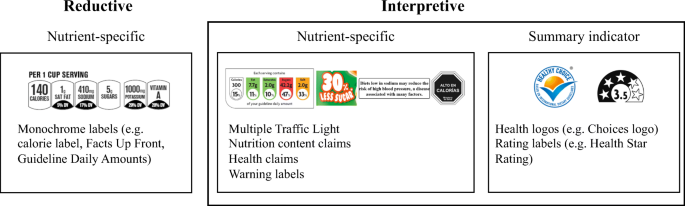
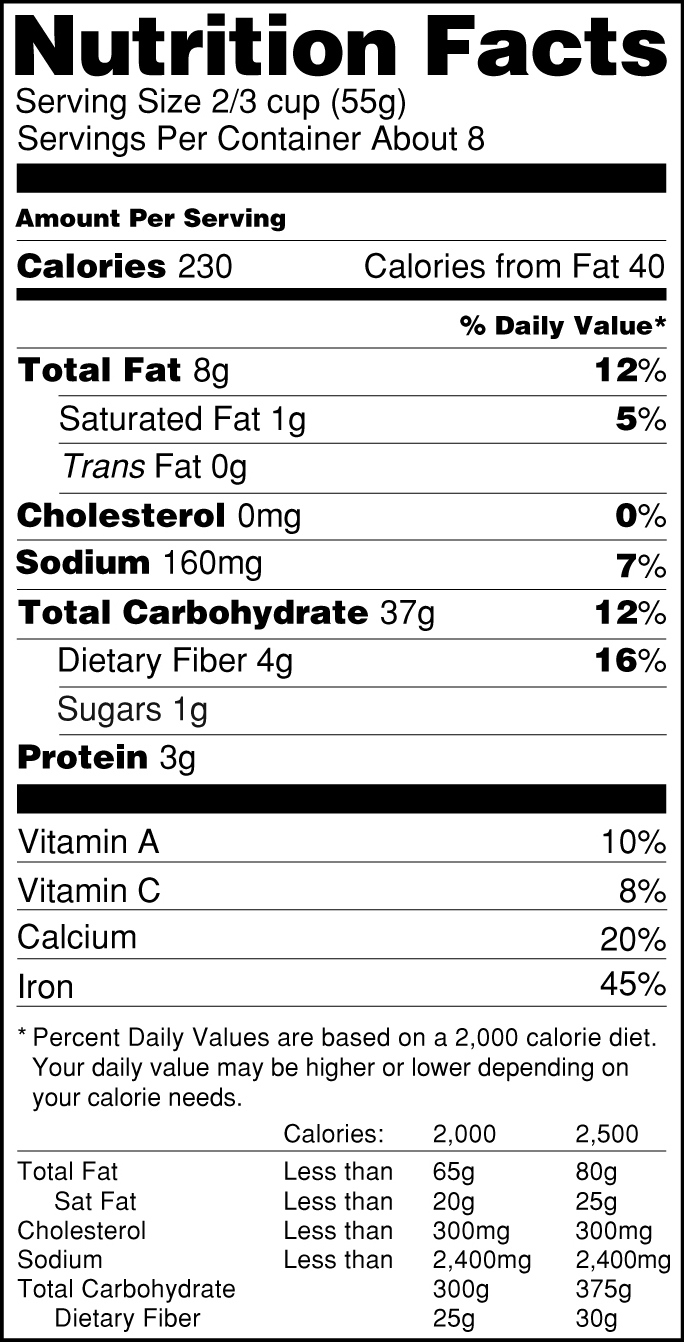
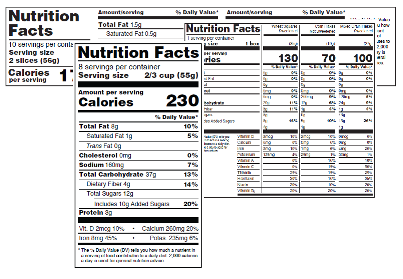
45 health claims on food labels are standardized and regulated
Background Information: Dietary Supplements - Consumer Drug manufacturers may claim that their product will diagnose, cure, mitigate, treat, or prevent a disease. Such claims may not legally be made for dietary supplements. The label of a dietary supplement or food product may contain one of three types of claims: a health claim, nutrient content claim, or structure/function claim. Health claims ... Nutrition facts label - Wikipedia The Ministry of Health and Family Welfare had, on September 19, 2008, notified the Prevention of Food Adulteration (5th Amendment) Rules, 2008, mandating packaged food manufacturers to declare on their product labels nutritional information and a mark from the F.P.O or Agmark (Companies that are responsible for checking food products) to enable ...
Prostate Cancer, Nutrition, and Dietary Supplements (PDQ ... Companies distribute calcium as a dietary supplement. In the United States, dietary supplements are regulated as foods, not drugs. Therefore, premarket evaluation and approval of such supplements by the U.S. Food and Drug Administration (FDA) are not required unless specific disease prevention or treatment claims are made. The FDA can remove ...

Health claims on food labels are standardized and regulated
Using Dietary Supplements Wisely | NCCIH However, some types of claims related to health or the way that the product affects the structure or function of the body may appear on dietary supplement labels. Health claims describe a relationship between a substance in the supplement and reduced risk of a disease or condition. They must be based on scientific evidence. Food/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · Labeling & Nutrition: Food Labeling: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, and Health Claims Small Entity Compliance Guide (August 2003) Questions and Answers on Dietary Supplements | FDA May 06, 2022 · Among the claims that can be used on dietary supplement labels are three categories of claims that are defined by the FD&C Act and FDA regulations: health claims (claims about the relationship ...
Health claims on food labels are standardized and regulated. Federal Register :: National Bioengineered Food Disclosure ... Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. Questions and Answers on Dietary Supplements | FDA May 06, 2022 · Among the claims that can be used on dietary supplement labels are three categories of claims that are defined by the FD&C Act and FDA regulations: health claims (claims about the relationship ... Food/Dietary Supplement Guidance and Regulatory Information Sep 30, 2022 · Labeling & Nutrition: Food Labeling: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, and Health Claims Small Entity Compliance Guide (August 2003) Using Dietary Supplements Wisely | NCCIH However, some types of claims related to health or the way that the product affects the structure or function of the body may appear on dietary supplement labels. Health claims describe a relationship between a substance in the supplement and reduced risk of a disease or condition. They must be based on scientific evidence.






![PDF] Nutrition labels and health claims: the global ...](https://d3i71xaburhd42.cloudfront.net/f8e40d7b317600da54b78934835b7ec21cd9b57d/24-Table1-1.png)



![PDF] Nutrition marketing on processed food packages in Canada ...](https://d3i71xaburhd42.cloudfront.net/6273f6117a666d80f88bc9641ac54dbfc8f285c5/2-Table1-1.png)


:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652082/healthy-choice.0.png)


















Post a Comment for "45 health claims on food labels are standardized and regulated"