41 fda guidance use of symbols on labels
Guidance for Industry and Food and Drug Administration Staff; Use of ... The guidance document entitled "Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use" provides guidance on the use of those recognized symbols. FDA announced the availability of the level 1 draft guidance document in the Federal Register of October 28, 2003 ( 68 FR 61449 ). Use of Symbols in Labeling | FDA Use of Symbols in Labeling The Food and Drug Administration (FDA) issued a final rule, Use of Symbols in Labeling, June 15, 2016, that became effective September 13, 2016. The final rule permits...
› scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ...
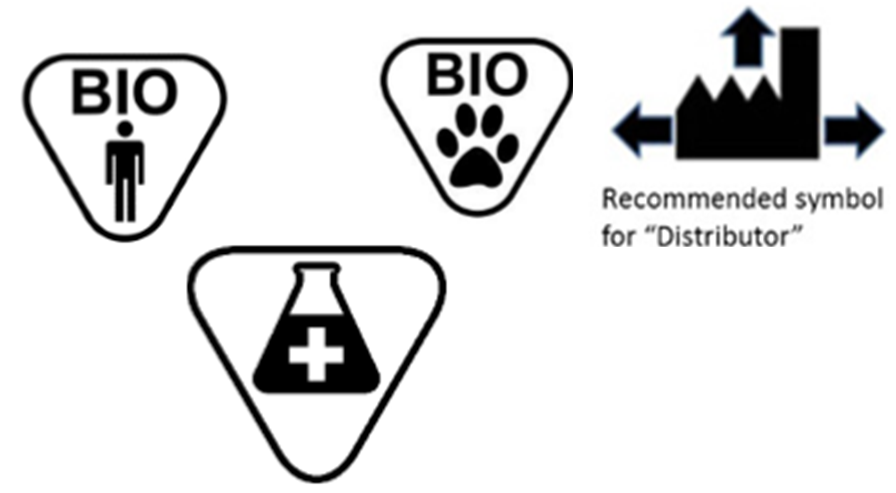
Fda guidance use of symbols on labels
› current › title-21eCFR :: 21 CFR Part 101 -- Food Labeling (11) If a product is promoted on the label, labeling, or advertising for a use that differs in quantity by twofold or greater from the use upon which the reference amount in § 101.12(b) was based (e.g., liquid cream substitutes promoted for use with breakfast cereals), the manufacturer shall provide a second column of nutrition information ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Jul 20, 2022 · The words intended uses or words of similar import in §§ 801.5, 801.119, 801.122, and 1100.5 of this chapter refer to the objective intent of the persons legally responsible for the labeling of an article (or their representatives). The intent may be shown by such persons' expressions, the design or composition of the article, or by the circumstances surrounding the … PDF Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Device ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553.
Fda guidance use of symbols on labels. EOF › regulatory-information › search-fdaGuidance for Industry, Q7A Good Manufacturing Practice ... Sep 24, 2001 · I. INTRODUCTION (1) A. Objective (1.1) This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs ... Guidance for Industry, Q7A Good Manufacturing Practice Guidance … Sep 24, 2001 · I. INTRODUCTION (1) A. Objective (1.1) This document is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs ... › regulatory-information › search-fdaGuidance for the Use of Bayesian Statistics in Medical Device ... Feb 05, 2010 · You may also send an e-mail request to CDRH-Guidance@fda.hhs.gov to receive a copy of the guidance. Please use the document number 1601 to identify the guidance you are requesting.
Federal Register :: National Bioengineered Food Disclosure Standard Dec 21, 2018 · Section 66.3(a) requires that labels for bioengineered food must bear a BE disclosure consistent with the requirements of part 66. Section 66.3(a)(2) prohibits labels for food that is not bioengineered from bearing a BE disclosure unless the food may bear a voluntary disclosure under § 66.116, based on records maintained under § 66.302. FDA ANNOUNCES GUIDANCE FOR SYMBOL USE ON IVD LABELS - FDAnews The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices. › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims |FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ... TaqPath COVID-19 Combo Kit -Instructions for Use For descriptions of symbols on product labels or product documents, ... FDA-released Instructions for Use with removal of the licensing statement covering Limited Use Label ... thermofisher.com ...
Small Entity Compliance Guide on Structure/Function Claims | FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ... Draft Guidance for Industry and FDA Staff; Use of Symbols on Labels and ... This document provides guidance on the use of selected symbols in place of text to convey some of the information required for in vitro diagnostic devices (IVDs) intended for professional use by FDA's labeling requirements for IVDs. This draft guidance is not final nor is it in effect at this time. DATES: › media › 136112TaqPath COVID-19 Combo Kit -Instructions for Use For descriptions of symbols on product labels or product documents, ... FDA-released Instructions for Use with removal of the licensing statement covering Limited Use Label ... thermofisher.com ... Use of the Term Healthy on Food Labeling | FDA Sep 28, 2022 · The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance.
› food › food-labeling-nutritionUse of the Term Healthy on Food Labeling | FDA Sep 28, 2022 · The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance.
Special - mowa.cheaty-do-pobrania.pl Aug 22, 2013. FDA approved the use of electronic labelling for prescription medical devices intended for use in U.S. healthcare facilities in 2003. 1 In the European Union, guidance on providing electronic instructions for use (EIFUs) for in vitro diagnostic devices has been available since 2007. 2 In March 2012, the EU Commission published.
eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ...
This document specifies - ndyzxa.fwpkrynica.pl This document specifies symbols used to express information supplied for a medical device.This document is applicable to symbol s used in a broad spectrum of medical devices, that are available globally and need to meet different regulatory requirements.EN ISO 15223-1:2012: Medical devices Symbols to be used with medical device labels, labeling, and information to be supplied - Part 1 ...
Guidance for the Use of Bayesian Statistics in Medical Device Clinical Feb 05, 2010 · You may also send an e-mail request to CDRH-Guidance@fda.hhs.gov to receive a copy of the guidance. Please use the document number 1601 to identify the guidance you are requesting.
Food/Dietary Supplement Guidance and Regulatory Information May 16, 2022 · WITHDRAWN Acidified and Low-Acid Canned Foods: (DRAFT) Submitting Form FDA 2541 (Food Canning Establishment Registration) and Forms FDA 2541d, FDA 2541e, FDA 2541f, and FDA 2541g (Food Process ...
Fda Announces Guidance for Symbol Use on Ivd Labels The FDA published a final guidance Nov. 29 outlining recommendations on the use of symbols on labeling for in vitro diagnostic devices (IVDs) to harmonize standards of conveying pertinent and required information about the devices.
Nutrition facts label - Wikipedia The nutrition facts label (also known as the nutrition information panel, and other slight variations) is a label required on most packaged food in many countries, showing what nutrients and other ingredients (to limit and get enough of) are in the food. Labels are usually based on official nutritional rating systems.Most countries also release overall nutrition guides for general …
FDA Issues Final Rule Permitting Use of Symbols on Device Labeling Three years ago, FDA proposed a rule that would permit device manufacturers to use stand‑alone symbols on device labeling. In the meantime, this issue has continued to fester. So it is good news that FDA finalized the rule on June 15, 2016. Under the final rule, device manufacturers may use symbols on device labeling in one of the following ways:
PDF Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Device ... Guidance for Industry and FDA Staff Use of Symbols on Labels and in Labeling of In Vitro Diagnostic Devices Intended for Professional Use Document issued on: November 30, 2004 The draft of this document was issued on October 28, 2003. The information collection provisions in this guidance have been approved under OMB control number 0910-0553.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Jul 20, 2022 · The words intended uses or words of similar import in §§ 801.5, 801.119, 801.122, and 1100.5 of this chapter refer to the objective intent of the persons legally responsible for the labeling of an article (or their representatives). The intent may be shown by such persons' expressions, the design or composition of the article, or by the circumstances surrounding the …
› current › title-21eCFR :: 21 CFR Part 101 -- Food Labeling (11) If a product is promoted on the label, labeling, or advertising for a use that differs in quantity by twofold or greater from the use upon which the reference amount in § 101.12(b) was based (e.g., liquid cream substitutes promoted for use with breakfast cereals), the manufacturer shall provide a second column of nutrition information ...

![PDF] Signs of change or clash of symbols? FDA regulation of ...](https://d3i71xaburhd42.cloudfront.net/103393b061f49e3976a4eaa8b35a160bfa9b2f8a/12-Figure1-1.png)
.png.aspx)




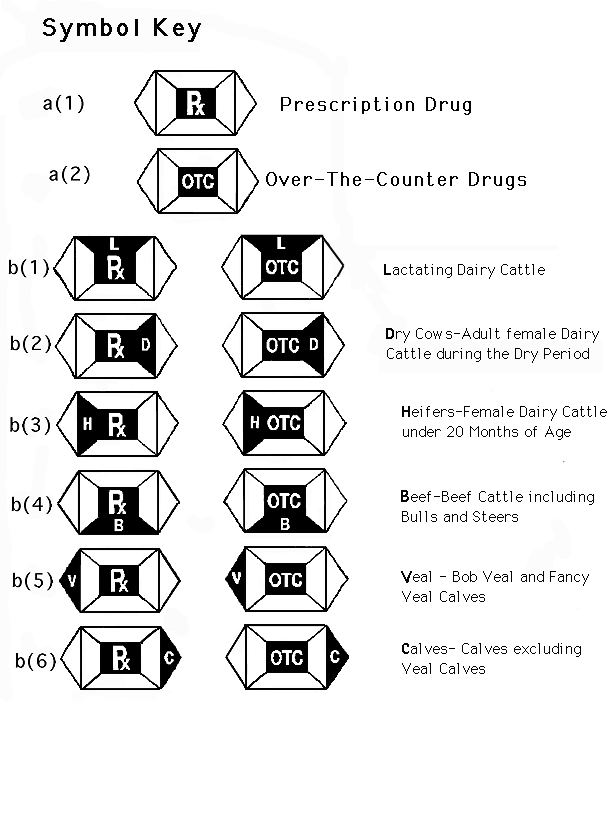
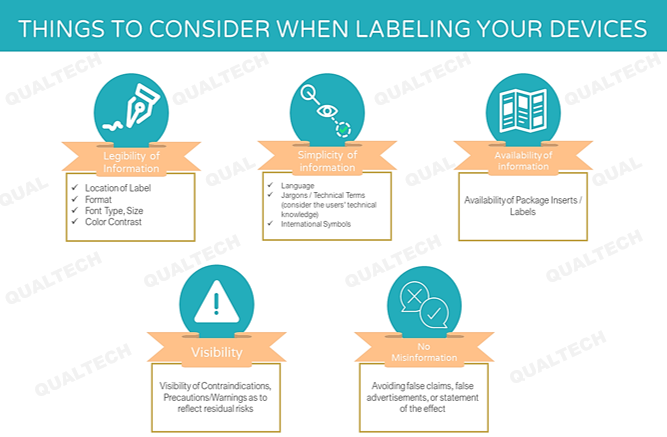

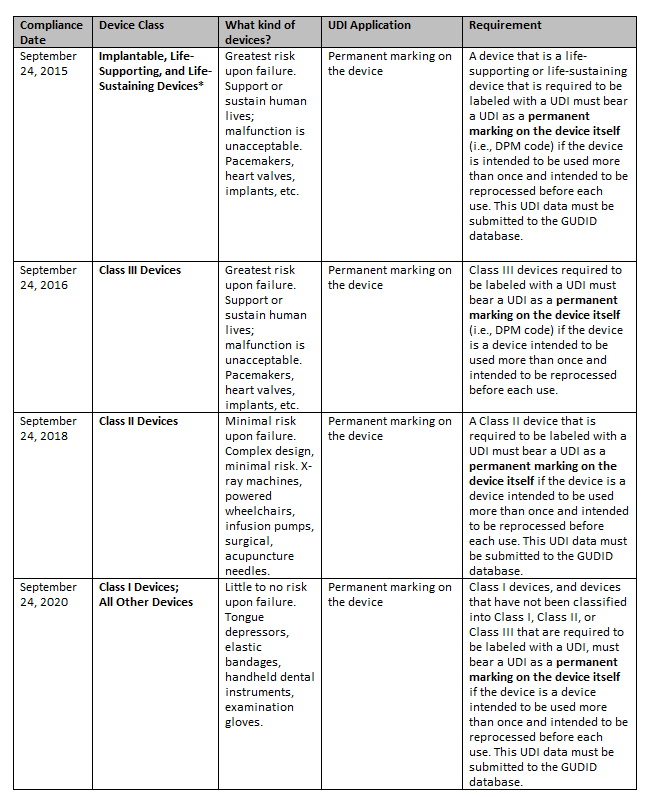


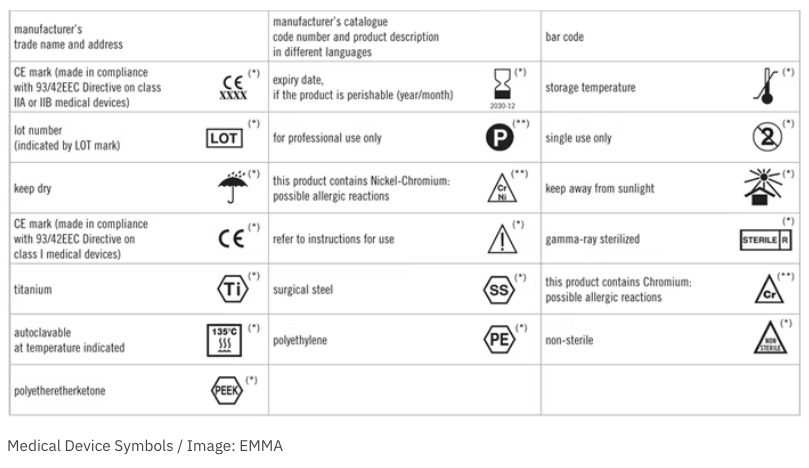
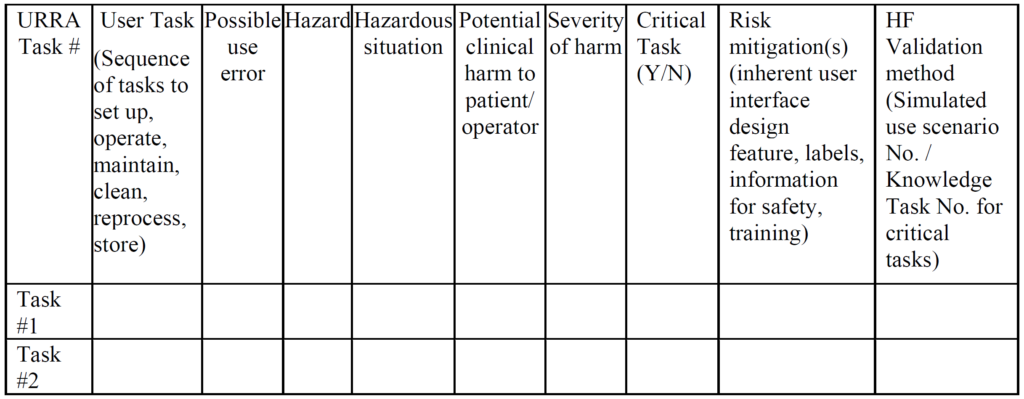

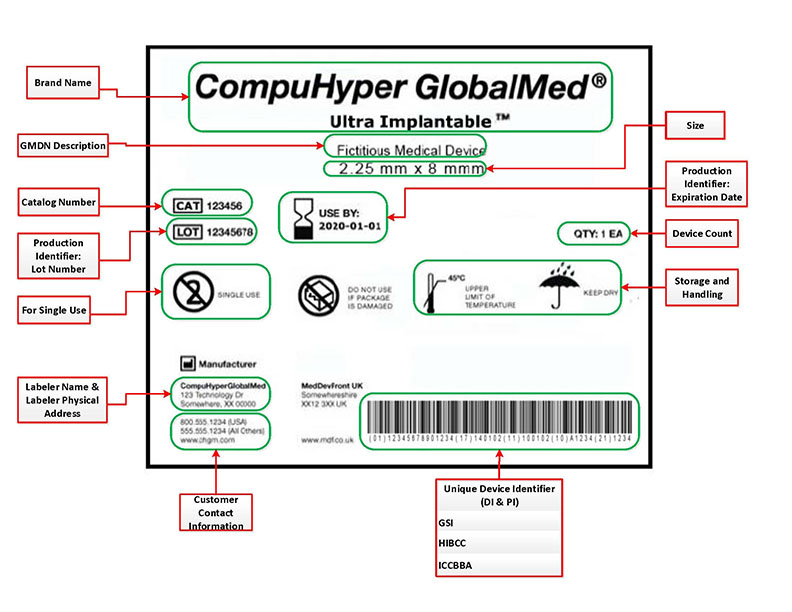








Post a Comment for "41 fda guidance use of symbols on labels"